How sucrose could transform gene therapy
Sugar holds the key to safer, more efficient gene-therapy, researchers have found, enabling new treatments for a broad range of disease conditions.
Sugar holds the key to safer, more efficient gene-therapy, researchers have found, enabling new treatments for a broad range of disease conditions.
A spoonful of sugar can indeed help the medicine go down, with researchers discovering that sucrose can be used to deliver safer, more efficient gene-based therapeutics.
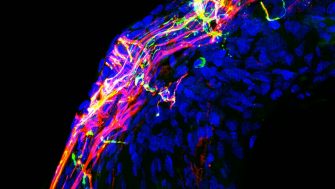
The addition of sucrose has improved a gene augmentation therapy used to regrow auditory nerves and improve hearing in animals, new research led by UNSW Sydney published in the journal of Advanced Science shows.
It also paves the way for DNA and RNA therapeutics to be used for treatments for a broader range of diseases, including brain disorders.
Researchers have taken advantage of the low electrical conductivity of sucrose to fine-tune an existing UNSW technology known as BaDGE® (a Bionic array Directed Gene Electrotransfer device) which creates a focused electric field, or electro-lens, to enable precision delivery of DNA and RNA to cells.
The technology has been used to deliver unpackaged DNA encoding nerve growth factors (neurotrophins) to the cochlea, in the inner ear, in deafened guinea pigs and cats. This has allowed the auditory nerve to regrow within a few weeks, improving long-term hearing in animals with cochlear implants.
First-in-human clinical trials are now underway using this technology, delivering gene therapy during cochlear implant surgery to regenerate auditory nerves so they better connect with the implant. The findings are expected to be published in the coming year.
“We discovered that if we used a sucrose-based carrier for the DNA - which displaced the salt that is normally around cells - then the electric field is much more efficient at delivering the negatively charged DNA to the cell membrane,” said UNSW Scientia Professor and report co-author Gary Housley, who is Chair of Physiology and director of the Translational Neuroscience Facility in the School of Biomedical Sciences.
“We could steer the therapeutic DNA exactly where we needed in the cochlea, which enabled nerve fibers to grow back out to be closer to a cochlear implant.”
Reducing local tissue conductivity also meant they could drastically reduce the amount of energy required to deliver the gene-based therapeutics.
“We were able to reduce the current in the electric pulses delivered to the cochleae in the animals by about 1000 times compared to the original research,” Prof. Housley said.
The method, dubbed ‘conductivity clamping’, makes the process significantly more efficient, but also reduces the risk of damage to sensitive tissue, like the brain, which would be harmed by the high levels of energy typically needed to achieve gene electrotransfer. So, the researchers took their work a step further.
“We were able to use this technology to achieve precise gene expression in the brain in guinea pigs,” Prof. Housley said.
“This research paves the way for the precision delivery of 'naked’ DNA/RNA relevant to new treatments for a broad range of conditions. It overcomes the drawbacks of current options, which can cause adverse immune reactions, have more limitations on their control and the size of DNA and mRNA molecules which can be used, and have prohibitive cost and development times.”
The research, led by the BaDGE® development group at UNSW, was conducted in collaboration with research and clinical team members from Macquarie University, The University of Sydney, The University of Melbourne, University of Tasmania, The Royal Prince Alfred Hospital, cochlear implant centre NextSense, and industry partner Cochlear Ltd. International collaborators included CNRS France and Université Paris Cité.
“This work is a tribute to collaborative strengths of universities and biotech industry,” Prof. Housley said.